The Possible Implications of Decision Memo for Next Generation Sequencing (NGS) for Medicare Beneficiaries with Advanced Cancer (CAG-00450R)
Lede
On January 27th, 2020, The Centers for Medicare & Medicaid Services (CMS) posted a National Coverage Determination (NCD) applicable to diagnostic lab tests using NGS for somatic (acquired) and germline (inherited) cancer. The full NCD is available here:
Highlights
The NCD is a final ruling after an initial NCD was proposed in late 2019, and CMS accepted feedback and ultimately addressed concerns. In the final analysis, four key takeaways appear likely:
1. The final NCD clarifies that Medicare contractors have discretion to make the § 1862(a)(1)(A) determination for all non-cancer diagnostic uses of NGS.
2. It was not CMS’ intent to restrict or remove coverage. CMS’ intent was to expand coverage. In the final NCD, we have clarified language to allow MAC discretion for any cancer diagnosis including tests that are not FDA approved or cleared for breast and ovarian cancer provided all the criteria is met. The final NCD also removed the diagnostic laboratory test criteria of the indication for NGS to be used for breast and ovarian cancer only.
3. This new NCD appears to overturn a prior NCD (or NCD proposal) that was introduced in March of 2018 with regard to early stage cancer coverage. The snippet below is from that NCD (our emphasis added).
"Patient has:
- either recurrent, relapsed, refractory, metastatic, or advanced stages III or IV cancer"
4. This appears to have expanded the market for applicable NGS companies to a much larger collection of patients -- namely those diagnosed with early stage cancer. Or, if those patients were already receiving coverage under the prior NCD, this new ruling then removes the risk that the prior proposed constraint would be imposed.
Definitions
Our final analysis was shared via Twitter and we reprise that micro blog summary below. The actual thread can be reviewed here: Twitter thread
First, there is some jargon we can work through:
CMS: The Centers for Medicare & Medicaid Services NCD: National coverage determination is a United States nationwide determination of whether Medicare will pay for an item or service.
MAC: Medicare Administrative Contractor (MAC) is a private healthcare insurer that has been awarded a geographic jurisdiction to process Medicare Part A and Medicare Part B medical claims.
Germline: Germline DNA refers to tissue derived from reproductive cells (egg or sperm) that become incorporated into the DNA of every cell in the body of the offspring aka 'inherited'
NGS: next-generation sequencing.
Analysis
Amid criticism that the policy was too narrow, the agency in 2019 opened consideration of widening coverage to earlier stage cancers.
Ruling: Simply put, CMS has widened the coverage area for germline cancer to all stages (stage 1 - stage 4). Prior proposals limited coverage to late stage (stages 3 and 4). Images 1 and 2 (below) are directly from the new NCD. The final image is a reprise of the image from the prior NCD that went into effect in March 2018.

"The final NCD clarifies that Medicare contractors have discretion to make the § 1862(a)(1)(A) determination for all non-cancer diagnostic uses of NGS."

"It was not CMS’ intent to restrict or remove coverage. CMS’ intent was to expand coverage. In the final NCD, we have clarified language to allow MAC discretion for any cancer diagnosis including tests that are not FDA approved or cleared for breast and ovarian cancer provided all the criteria is met. The final NCD also removed the diagnostic laboratory test criteria of the indication for NGS to be used for breast and ovarian cancer only."
And then a snippet from the 2018 NCD.

"Patient has:
- either recurrent, relapsed, refractory, metastatic, or advanced stages III or IV cancer"
While 'Part A' of the CMS ruling restricts testing coverage to FDA approved or cleared germline NGS tests, there are no such tests in existence that meet all of the criteria * Germline * NGS * FDA cleared
This could, initially, be mistaken as a reduction in coverage given the FDA requirement. That's not quite right.
The critical takeaway was not 'Part A,' but rather 'Part B' which focuses on greater discretion for MACs. Currently there are 12 A/B MACs and 4 DME MACs in the program that process Medicare FFS claims for nearly 70% of the total Medicare beneficiary population.
Part A is the CMS' rule of absolute 'must be covered' tests - i.e. a minimum population that must be covered. But, it's the MACs that have been given discretion to expand that minimum population. Here is a useful source: CMS widens NGS test coverage for inherited breast, ovarian cancers
Part B (see image below) has given MACs the discretion to cover * any cancer diagnosis (any cancer, any stage).
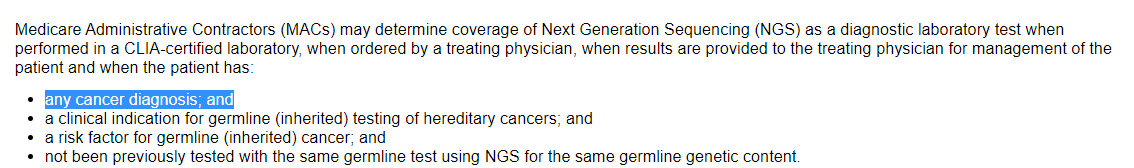
Implications
According to breastcancer.org, in 2020, an estimated 276,480 new cases of invasive breast cancer are expected to be diagnosed in women in the U.S., along with 48,530 new cases of non-invasive (in situ) breast cancer.
According to the ovarian cancer research alliance (OCRA), there were 22,530 new cases of ovarian cancer diagnosed in 2019 and, regrettably, 13,980 estimated deaths.
In my opinion, CMS' final NCD has cleared the path to more testing coverage for early stage ovarian and breast cancer, as well as other germline cancer diagnoses.
If we make an assumption that roughly half of germline cancer diagnoses are early (there are reasons to argue against this), then this decision has cleared the way for doubling the amount of people that can be tested and, ultimately, see improved outcomes.
I am not a CMS expert by any stretch, and if someone on this thread is, please feel free to chime in.
The author is long shares of Invitae (NYSE:NVTA) at the time of this writing.
Thanks for reading, friends.
Legal
The information contained on this site is provided for general informational purposes, as a convenience to the readers. The materials are not a substitute for obtaining professional advice from a qualified person, firm or corporation. Consult the appropriate professional advisor for more complete and current information. Capital Market Laboratories (“The Company”) does not engage in rendering any legal or professional services by placing these general informational materials on this website.
The Company specifically disclaims any liability, whether based in contract, tort, strict liability or otherwise, for any direct, indirect, incidental, consequential, or special damages arising out of or in any way connected with access to or use of the site, even if we have been advised of the possibility of such damages, including liability in connection with mistakes or omissions in, or delays in transmission of, information to or from the user, interruptions in telecommunications connections to the site or viruses.
The Company makes no representations or warranties about the accuracy or completeness of the information contained on this website. Any links provided to other server sites are offered as a matter of convenience and in no way are meant to imply that The Company endorses, sponsors, promotes or is affiliated with the owners of or participants in those sites, or endorse any information contained on those sites, unless expressly stated.


