The Cancer Focused Biotech Gem Institutions are Hiding
Bellicum Pharmaceuticals
Written by Ophir Gottlieb, 1-19-2015Follow @OphirGottlieb
PREFACE
Bellicum Pharmaceuticals (BLCM) is young biotech with explosive potential that does not rely on single drug, rather an entire technology. If you're looking for the few gems in biotech that have been beaten up simply because the market has tumbled, this may be one of them.
And here's another little known secret: Over 60% of the stock is owned by institutions because the top 1% know exactly what we're about to introduce and they don't want anyone else to know. Get ready for a ride.
This is a small cap clinical stage biotech with potentially world changing treatments in the laboratory. The stock has been absolutely trounced in the market correction and is now trading at an all-time low.
The resulting stock drop has created a $299 million market cap firm, with $110 million in cash and one of the richest pipelines and most innovative medical treatments coming through trials we have ever seen with respect to the treatment of cancer.
STORY
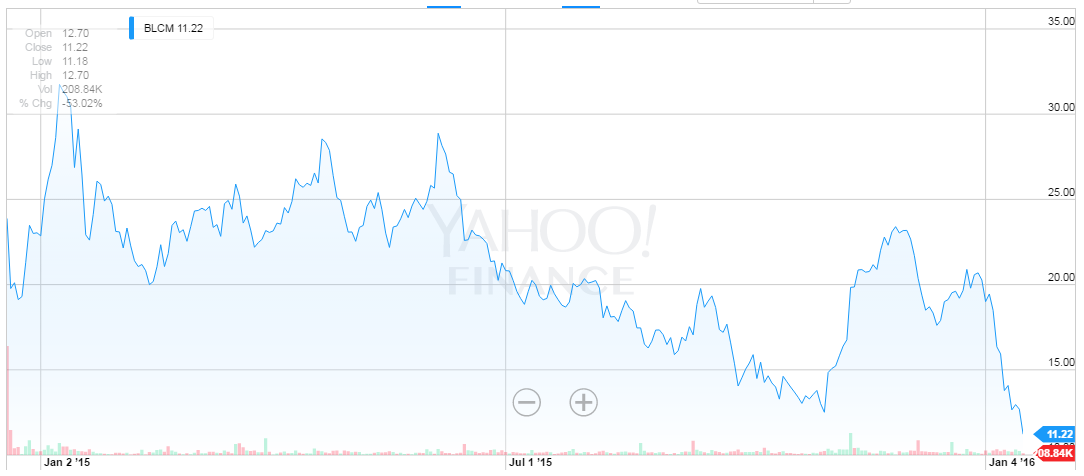
While the risk of failure can't be ignored, this is also a company with enormous potential upside and a new release last week has yet more exciting revelations. The company has announced regulatory milestones and program updates on its CAR-T and TCR product candidates. Before we get into the story, here's the all-time stock chart.
NEWS
On January 11th the company announced its submission of BPX-701 and BPX-601 Clinical Trial Protocols for Review by the NIH RAC. That means nothing right now, so let's get into it.
BLCM is in clinical trials to treat both cancer and genetic blood diseases and it has a fascinating story behind it. The firm is trying to to craft next-generation therapies that empower the immune system to battle back against cancer and other rare blood disorders. Two if its critical trials are for products with unassuming names BPX-501 and BPX-601.
On Saturday, December 5th, BLCM reported some high quality results from clinical trials. Here's what we know from that release:
BPX-501
BPX-501 consists of T cells taken from a stem cell donor and "modified in a lab plus a molecular safety switch to turn off the modified T cells in the event of serious graft-versus-host disease" (Source: TheStreet.com). The great marvel of BPX-501, if it works, is that it will enable donors to save children's lives: it's a bridge to allow family members to save each others' lives.
A study enrolled 39 children and all of them underwent a haploidentical, T cell-depleted stem cell transplant. That's medical speak for transplants from donors with stem cells which are not an exact match to the patient (most of them were from family members). Mismatched stem cells can cause infections and something called graft-versus-host disease (or GVHD), both of which are potentially fatal. This is where BPX-501 comes in.
Two weeks after the transplants, each of the children were infused with the trial drug and then data was compared to how these children did with how other children did that did not receive BPX-501. Here are the results:
On average, the immune system in patients treated with BPX-501 recovered 40 days faster and they were discharged from the hospital 21 days sooner than patients in the historical control.
None of the BPX-501 patients died from transplant-related causes to date compared with three deaths from transplant in the historical control.
The incidence GVHD, the most frequent and potentially deadly side effect of mismatched stem cell transplants, was also reduced by BPX-501 compared to historical controls.
None of the BPX-501 patients died from transplant-related causes to date compared with three deaths from transplant in the historical control.
The incidence GVHD, the most frequent and potentially deadly side effect of mismatched stem cell transplants, was also reduced by BPX-501 compared to historical controls.
So basically, in this interim trial, the treatment did quite well. The "safety switch" built into BPX-501 was never used because no severe cases of GVHD occurred. While we can all read these results, I think the most useful thing is to grab some quotes from doctors that focus on these diseases for a living, so here we go.
Dr. Franco Locatelli from Italy's IRCCS Ospedale Pediatrico Bambino Gesù said, "these interim results present strong evidence that the addition of BPX-501 modified donor T cells provides important immune support and improves outcomes in patients undergoing a T cell-depleted haploidentical stem cell transplant."
Do you enjoy discovering new companies and opportunities and really understanding them? Get Our (Free) News Alerts Once a Day.
THE OPPORTUNITY
It turns out that these haploidentical, T cell-depleted stem cell transplants are pretty rare, making up just 9% of total stem cell transplants every year -- that would be just over 2,000 of these transplants a year. As a stand alone business, this may truly be too small to matter in an economic sense. But, the potential goes much further.
A strong and thoughtful argument is being made that if BPX-501 really does work, then doctors will opt for these "mismatched" stem cell donor operations more often. That is a hypothesis founded on solid ground. But there's actually more.
This year, Bellicum plans to meet with the FDA and European drug regulators to determine the steps necessary to seek approval for BPX-501 in transplant patients with non-malignant disease. One example the Bellicum's COO gave surrounded beta-thalassemia, a blood disorder that reduces the production of hemoglobin.
The media isn't equipped to understand the stock market beyond headlines. Try CML Pro for free and watch yourself become the expert in the room. Try CML Pro. No credit Card. No Payment Info. Just the Power.
Now, Beta thalassemia is a fairly common blood disorder worldwide. In fact, thousands of infants are born each year with the affliction according to the National Institute of Health (NIH). That expands the possible usage of BPX-501, and also puts it head to head with Bluebird Bio (BLUE), which has a gene therapy to treat the disease.
We break news like this everyday. Discover the Undiscovered. Get Our (Free) News Alerts Once a Day.
MORE OPPORTUNITY: CANCER & CAR-T
Bellicum believes that the technology platform BPX-501 is built on can also be used to develop treatments for cancer, and now we're in a totally different league of disease, both in terms of its effects on society and its commercial viability. In fact, BPX-501 and BPX-601 may go hand in hand with CAR-T therapies. For those of you in the biotech world focusing on cancer cures, this is the game changer.
I've covered CAR-T with JUNO and KITE, but a quick review goes like this:
CAR-T therapeutics require the removal of a cancer patient's immune cells, selection and activation of the cells, and gene transfer to train the cells to attack the patient's tumor. At that point, the cells are grown in an incubator until there are enough to put back into the patient."
(Source: Fool.com).
(Source: Fool.com).
In English, the goal is to reprograms a patient's T-cells — the kind that are supposed to fight disease — to seek and destroy only abnormal, cancerous lymph cells, not the healthy ones crucial for human life. You can see the reason for massive optimism, as this would be a cure for cancer, not a treatment.
THE PROBLEM WITH CAR-T
More and more studies are coming out that show CAR-T could be an end all cure all for cancer in patients with good reactions, but the negative side effects are turning out to be impossible to ignore for patients with bad reactions. These new revelations are threatening the entire CAR-T world and now the companies that have bet the house on it (KITE, JUNO) have to worry abut the side-effects.
THE SOLUTION
Bellicum is working on what they call "GoCAR-T Technology" which incorporates a "switch" that activates CAR T cells when triggered. This is the promise of Bellicum on a broader, billion dollar scale. Here's an illustration from the Bellicum website that shows how the company aims to improve the standard CAR-T process:

Do you thrive on understanding what's really going on in a company beyond headlines to find uncovered gems? Get Our (Free) News Alerts Twice a Week
While JUNO and KITE battle it out to be the first to bring CAR-T to market, Bellicum stands firmly in the middle, to help either (or both) make their treatments safer and better. This is what Bellicum's website reads:
We believe these studies together provide proof-of-principle that GoCAR-T technology may turn[] CAR T cell therapy from an uncontrollable, and largely unpredictable class into a more predictable therapy which can be adjusted to the patient's therapeutic window.
(Source: Bellicum Webiste).
(Source: Bellicum Webiste).
The product called BPX-601 is Bellicum's GoCAR-T product candidate for solid tumors. The company writes, "we are currently conducting preclinical studies for the treatment of solid tumors overexpressing the prostate stem cell antigen (PSCA) antigen. We have obtained positive proof-of-principle data in an animal pancreatic tumor model, which we believe validate BPX-601's activity and rimiducid's ability to modulate therapeutic effect.
And perhaps the most important part:
Although many product candidates are in development for these cancers, there are currently no approved products targeting prostate stem cell antigen (PSCA). We intend to file an IND for the initial indication of pancreatic cancer by the end of 2015.
The main stream media doesn't have the vocabulary to understand breaking technology and biotech. Get free news alerts from us and you will be the expert in the room. Get Our (Free) News Alerts.
BPX-701
As if BLCM didn't have enough in the laboratory, it's 701 product is also making waves -- a treatment for different types of untreated melanoma.
CONCLUSION
BLCM is moving down the line with positive results for multiple treatments and a careful eye sees a massive opportunity with the "triggers" to make CAR-T safer. This is a young biotech with explosive potential that does not rely on single drug, rather an entire technology.
The risk is that the company fails and that would mean it could literally go to zero. The upside is equally fantastical. The firm has a market cap of $299 million, with $110 million in cash. If it wins with either or both of its treatments, this is a multi-billion dollar firm. These are the risk and reward scenarios for this fascinating biotech.
This is just one of the fantastic reports CML Pro members get. For a (very) limited time we are offering CML Pro at a 90% discount for $10/mo. with a lifetime guaranteed rate. Join Us: Get the Most Advanced Visual Tools, Data and Stock Research.
WHY THIS MATTERS
The top 1% are keenly aware of the deep data that will move markets. CML Pro has in depth research dossiers that select the number one top picks for breaking bioetch and technology: Artificial Intelligence, The Internet of Things, Cyber Security, Drones, Social Media and more.
CML research has been given the institutional stamp of approval sitting side by side with research from Goldman Sachs, Morgan Stanley, Barclay's and more on professional terminals. But while other institutional research can cost tens of thousands of dollars, CML Pro is being offered to our retail family for ten dollars for a limited time.
This is just one of the fantastic reports CML Pro members get along with all the charting tools, top picks for 2016, research dossiers and alerts. For a limited time we are offering CML Pro at a 90% discount for $10/mo. with a lifetime guaranteed rate. Join Us: Get the most advanced premium research delivered to your inbox along with access to visual tools and data that until now has only been made available to the top 1%.
Thanks for reading, friends.


